Introduction: In an open-label phase 2 study, blinatumomab demonstrated efficacy with a manageable safety profile as second salvage in patients with relapsed or refractory B-cell Non-Hodgkin's lymphoma (R/R B-NHL) following platinum-based salvage regimens (Coyle et al. Leukemia & Lymphoma. 2020: 1-10). Blinatumomab is a BiTE® (bispecific T-cell engager) immuno-oncology therapy that activates endogenous cytotoxic T cells to kill target B cells. Here, findings from the updated analysis are reported (NCT02910063).
Methods: Patients aged >18 years with biopsy-confirmed B-NHL without prior complete response or complete metabolic response (CMR) following first-line treatment with anthracycline- based chemotherapy and anti-CD20 therapy, had progressive metabolic disease (PMD), no metabolic response (NMR), or partial metabolic response (PMR; Lugano Classification) after ≥2 cycles of platinum-based S1 therapy were eligible. Blinatumomab was given by continuous intravenous infusion for a single 70-day cycle 1 (9 µg/day for 7 days, 28 µg /day for 7 days, and 112 µg /day for 42 days, followed by a 14-day treatment free interval) and an optional 28-day second cycle (9 µg /day for 7 days, 28 µg /day for 7 days and 112 µg / day for 14 days) at the investigator's discretion.
Primary endpoint was CMR by central PET. Additional endpoints included objective response rate (ORR; CMR plus PMR), overall survival (OS), progression- free survival (PFS), duration of response, post-response HSCT rate, and the incidence and severity of adverse events (AEs).
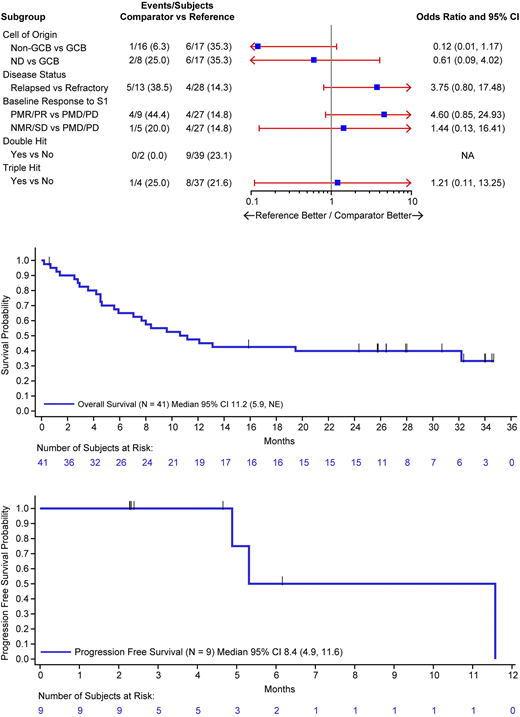
Results: As of the data cut date (June 3, 2020) for this updated analysis, 41 patients were enrolled between 23 January 2017 and 15 January 2018; 28 (68%) patients were refractory and 13 (32%) relapsed to first-line therapy, 66% had progressive disease following first salvage (S1), and 9 (22%) had double or triple hit status at baseline (Figure 1).
ORR was 37% (15/41; 95% CI, 22-53) after 12 weeks of treatment, including 9 (22%) patients who achieved CMR and 6 (15%) achieved PMR. Of the 41 patients enrolled, 17 (42%) were double refractory; of which, 3 (7%) achieved CMR, and 3 (7%) achieved PMR.
Of the 41 patients who received blinatumomab, median OS (95% CI) was 11.2 (5.9-NE) months with median follow-up time of 27.9 months. Among the 9 patients who achieved CMR, median OS (95% CI) was NE (7.0, NE) and median PFS (95% CI) was 8.4 (4.9-11.6) months with median follow of time of 4.7 months; of which, 3 patients had disease progression and 0 died. Of the 13 patients who achieved HSCT, median OS (95% CI) was NE (13.1-NE) with 69% of patients alive at 30 months and median PFS (95% CI) was 8.4 (5.3-13.9) months with 21% of patients alive at 12 months (Figure 2 and 3).
In total, 29 (71%) patients had grade ≥3 treatment-emergent AEs, including included infections (n=8; 20%), neutropenia (n=4; 10%), pulmonary embolism (n=1; 2%), and acute pancreatitis (n=1; 2%). Consistent with previous blinatumomab reports, neurologic events (NEs) were reported in 23 (56%) patients, including 10 (24%) with grade 3 NEs and 3 (7%) with NEs leading to treatment discontinuation. Grade 3 cytokine release syndrome was reported in only 1 patient. 7 (17%) patients discontinued treatment due to AEs and 7 (17%) had fatal AEs of which were related to disease progression.
Conclusions: In conclusion, durable complete remissions can be achieved with a manageable safety profile using blinatumomab as second salvage in patients with aggressive R/R B-NHL following platinum based first salvage regimens
Coyle:Amgen: Other: Travel support. Morley:Janssen: Honoraria, Other: Fees; AbbVie: Honoraria, Other: Fees; Roche: Other: travel support; Amgen: Honoraria, Other: Fees, travel support. Rambaldi:Sanofi: Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Omeros: Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support from Gilead.; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company). Advisory board and speaker fees from Pfizer.; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company). Research grant from Amgen Inc.; BMS/Celgene: Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Astellas: Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support of parent study and funding of editorial support. Received travel support., Research Funding; University of Milan: Current Employment. Furness:Amgen: Other: Travel Support. Desai:IQVIA: Current Employment. Mergen:Amgen: Current Employment, Current equity holder in publicly-traded company.
Durable complete remissions can be achieved with a manageable safety profile using blinatumomab as second salvage in patients with aggressive R/R B-NHL following platinum based first salvage regimens
Author notes
Asterisk with author names denotes non-ASH members.